Sleep Deprivation Increases Facial Skin Yellowness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Measurement of Skin Yellowness, Redness, and Hydration
2.4. Blood and Urine Sample Collection
2.5. HPLC Analysis
2.6. Statistical Analysis
3. Results
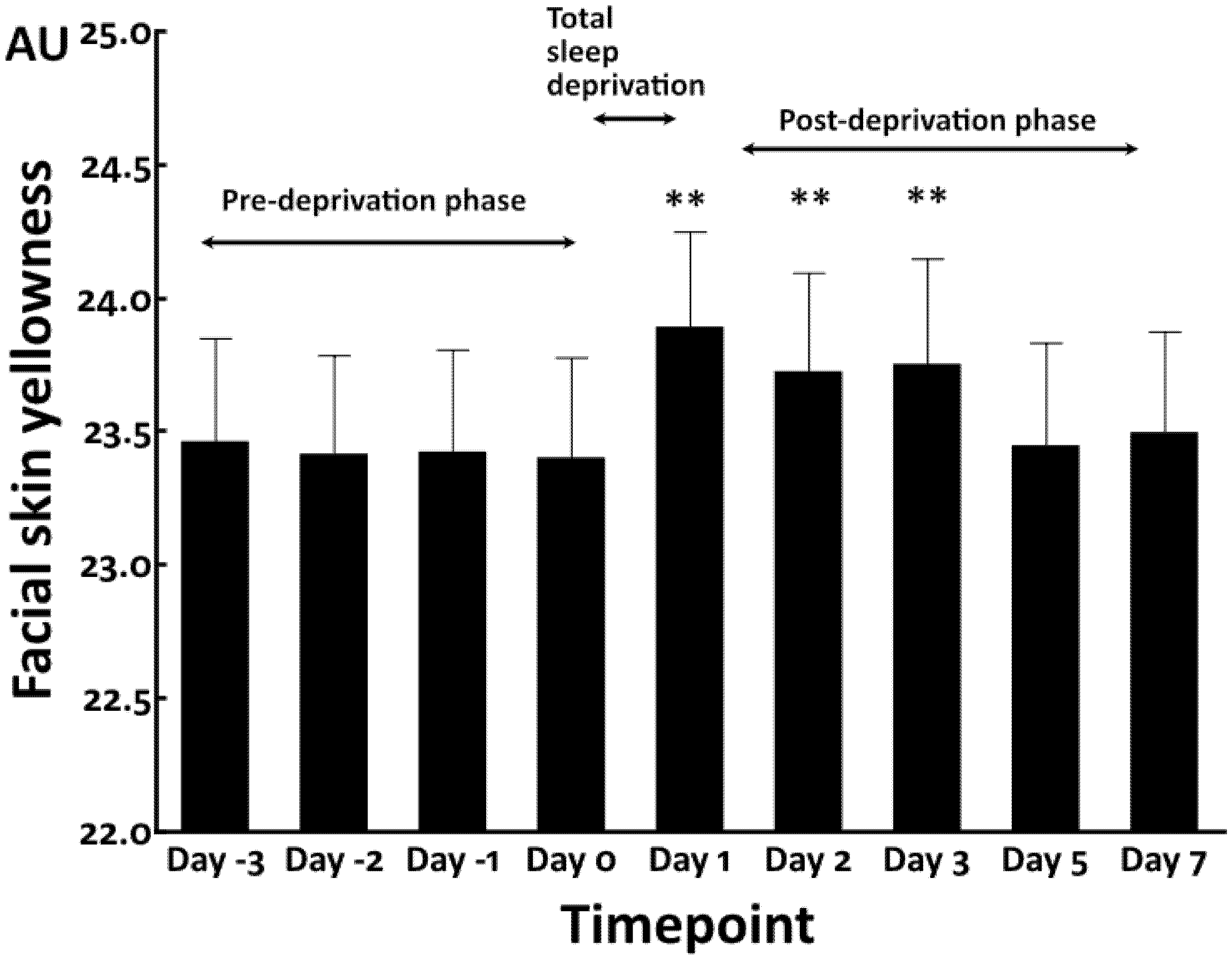
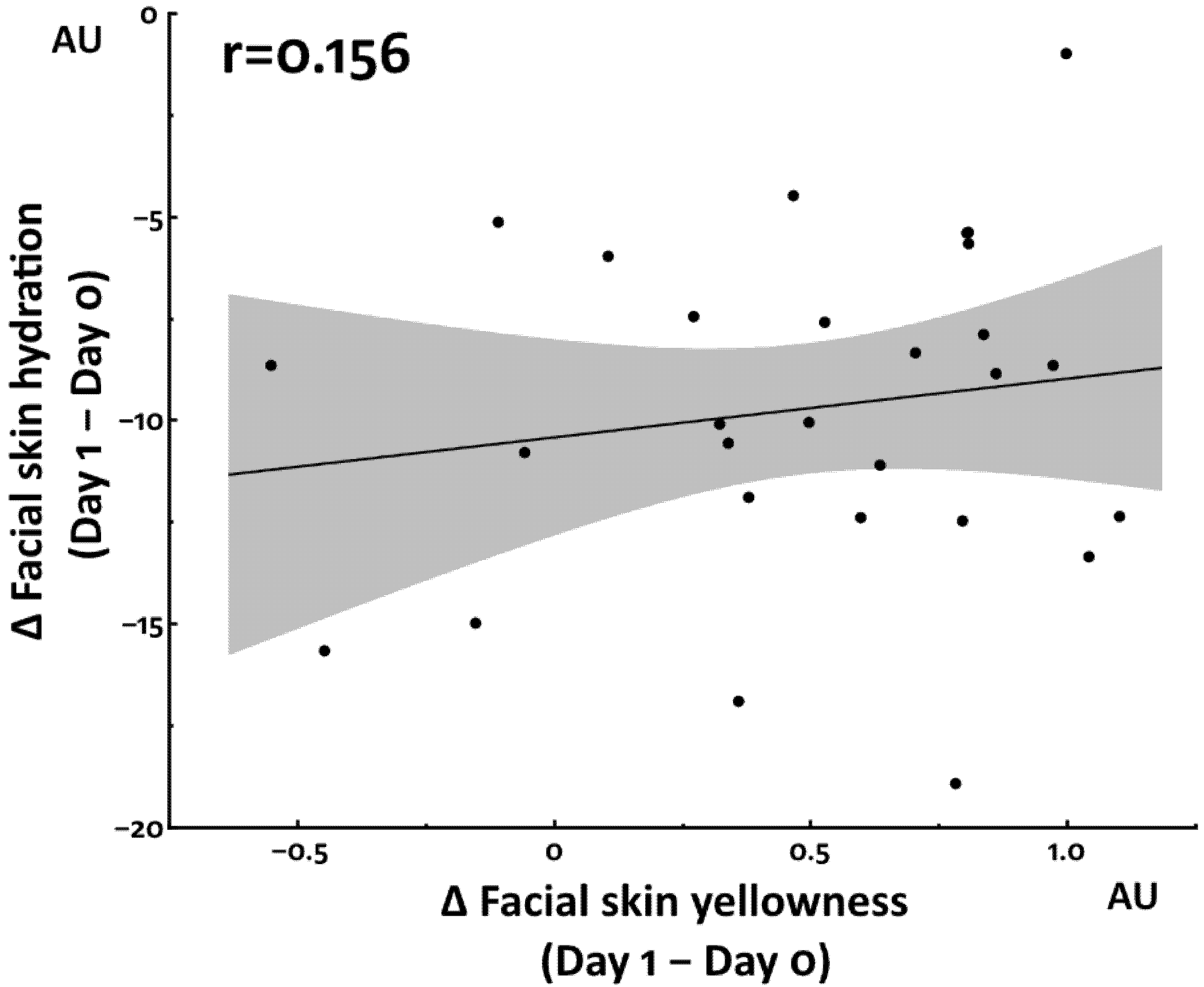
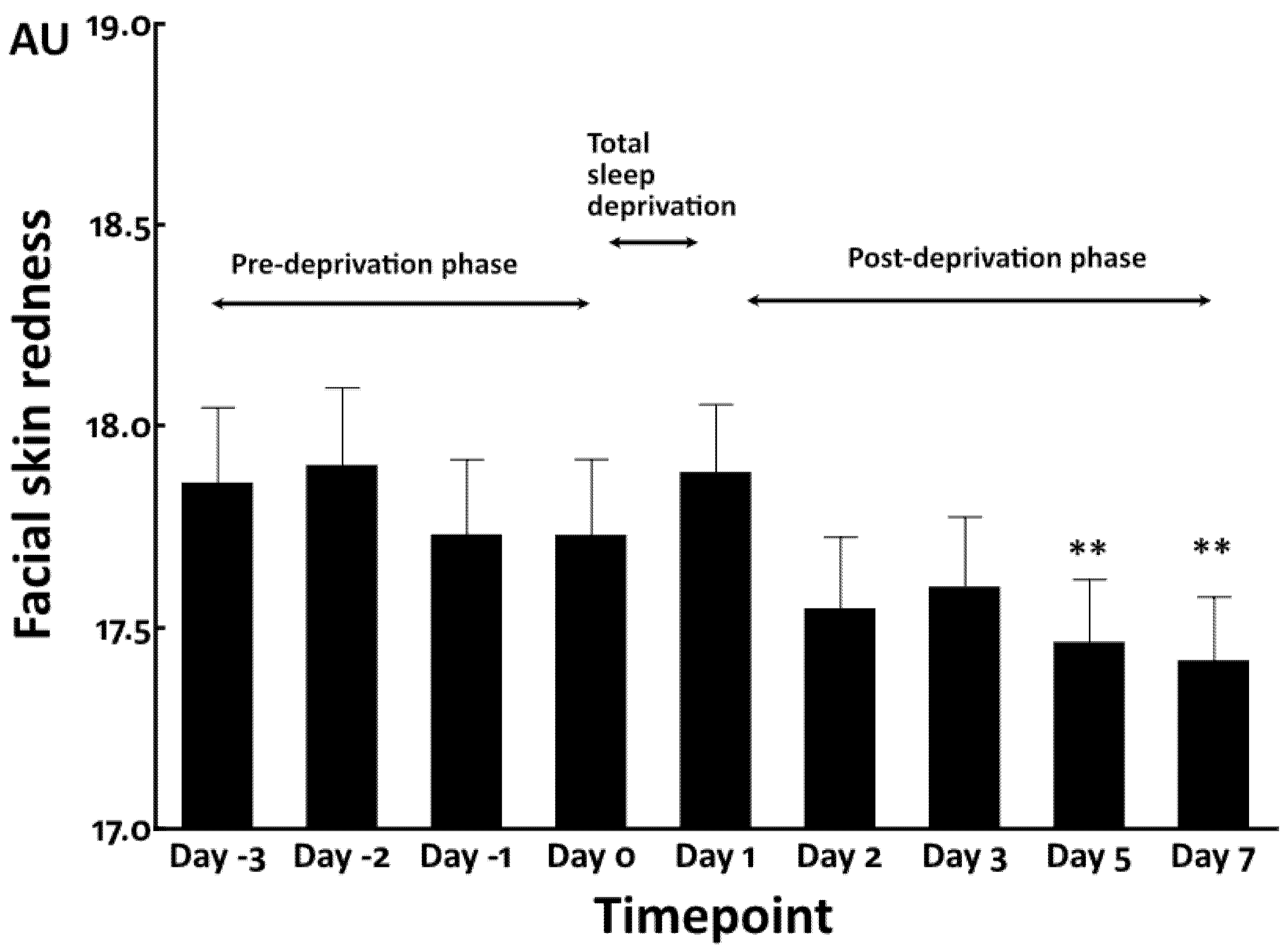
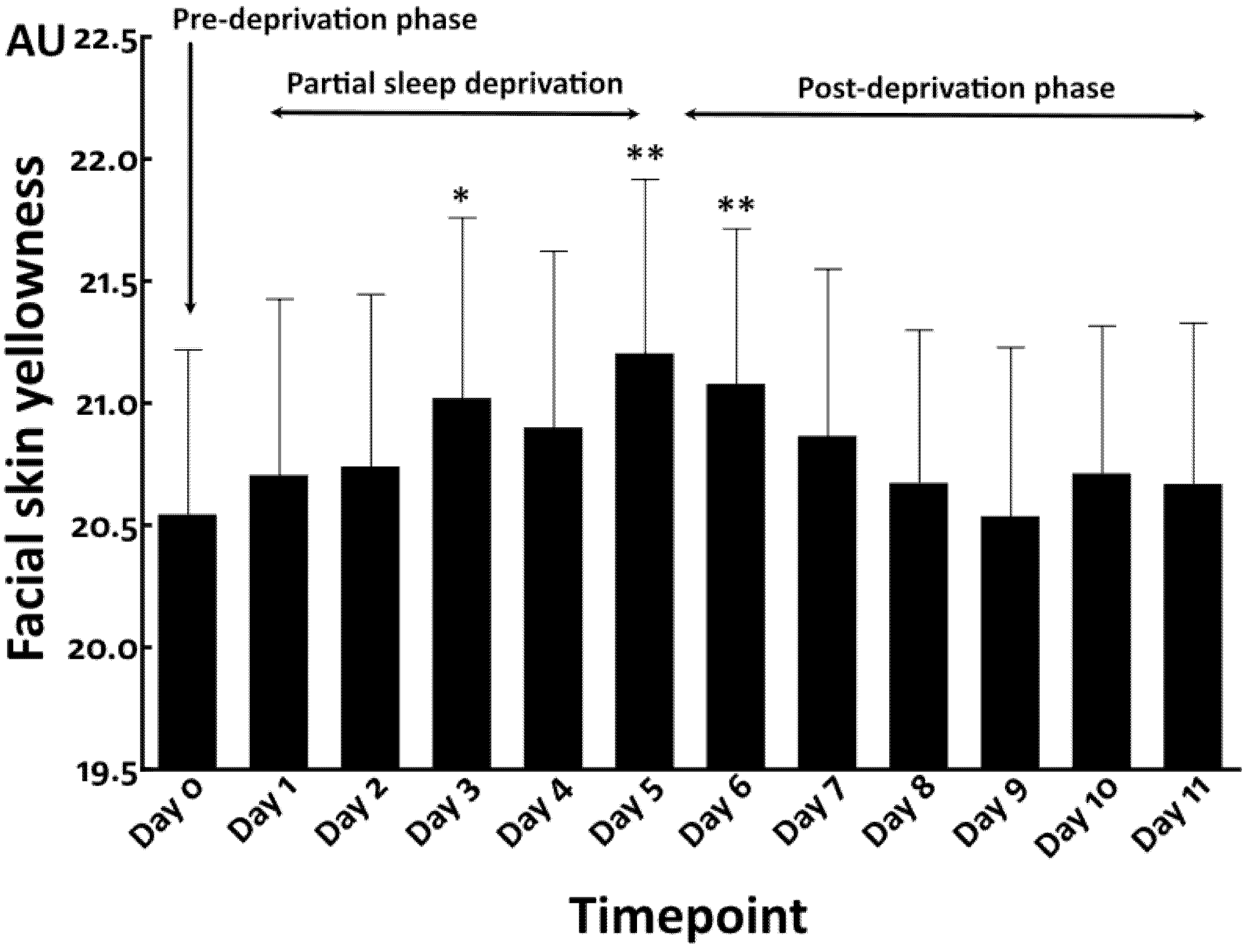
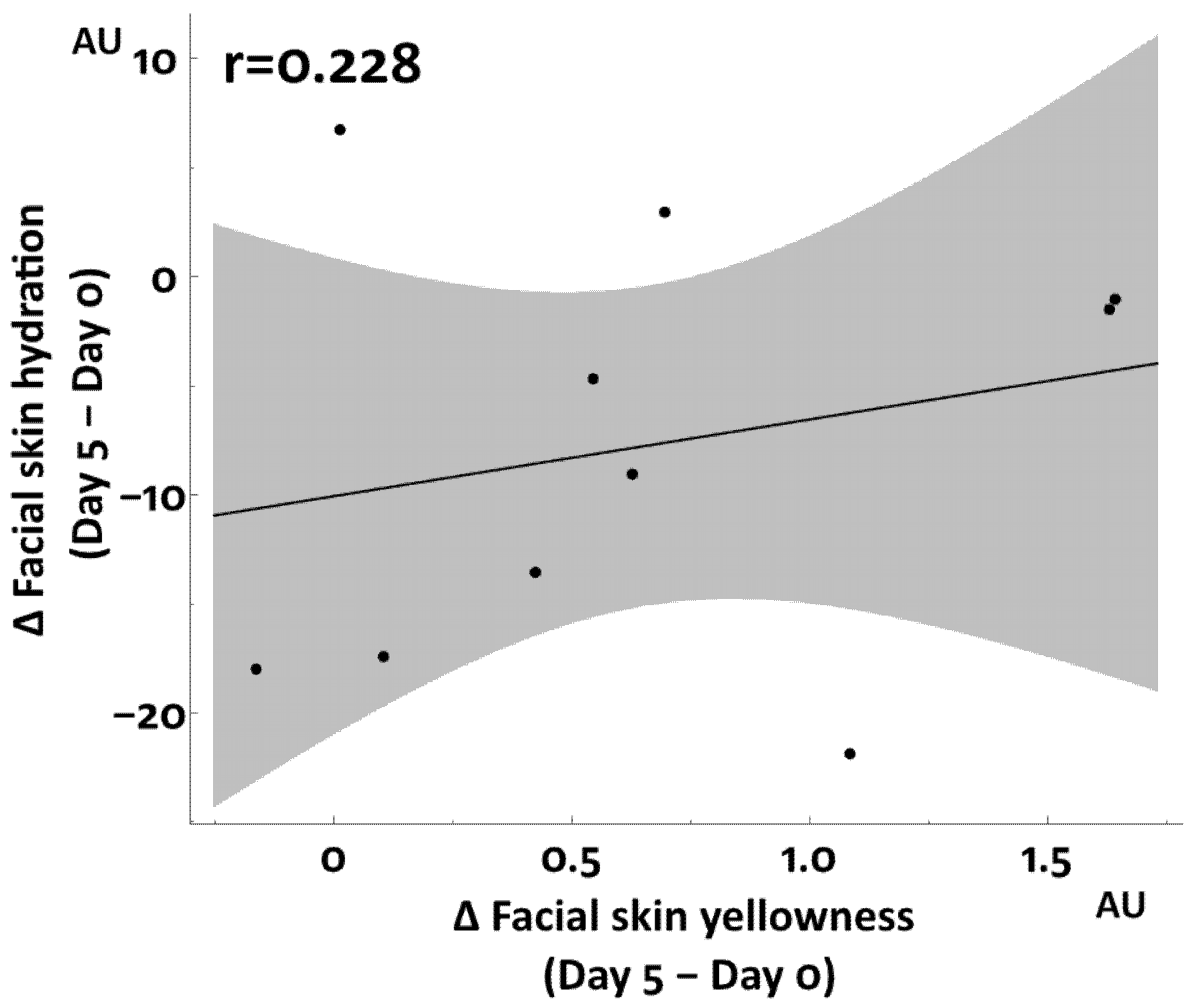
3.1. Increased Facial Skin Yellowness by Total Sleep Deprivation
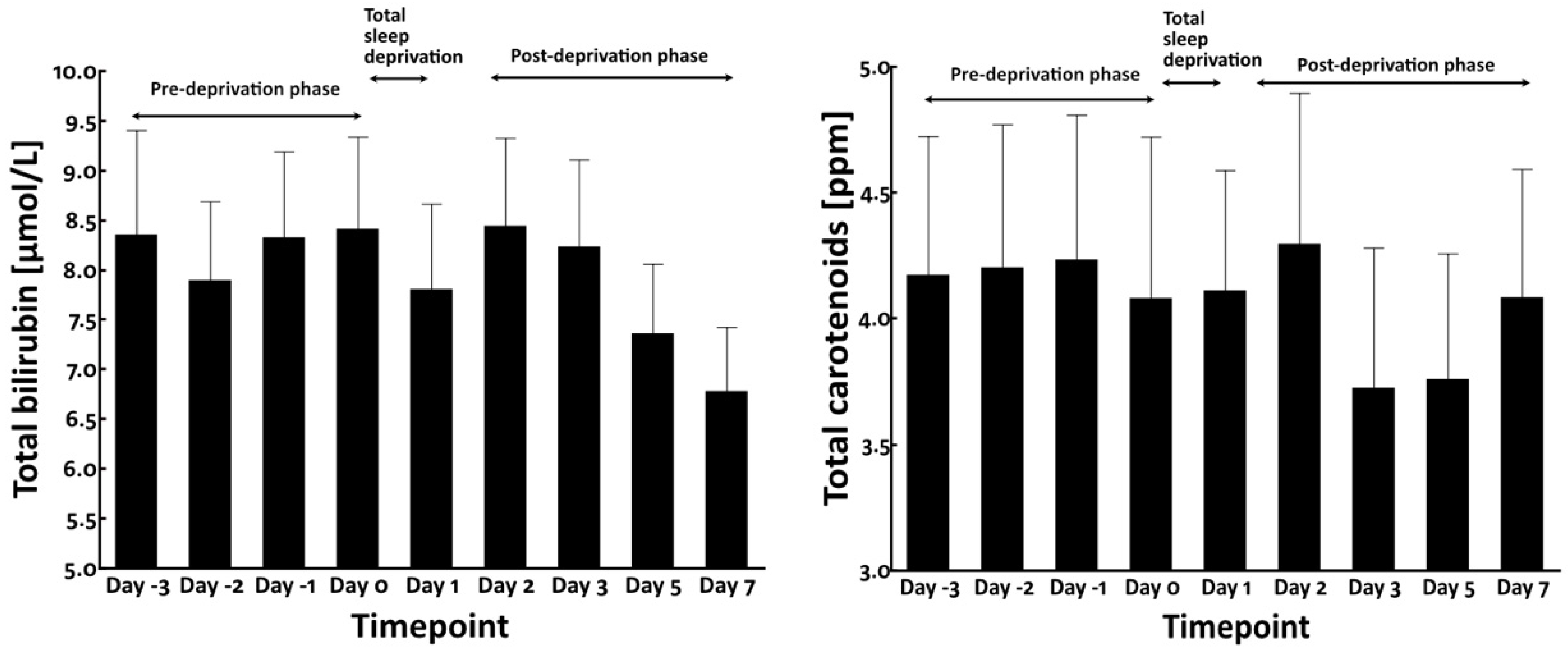
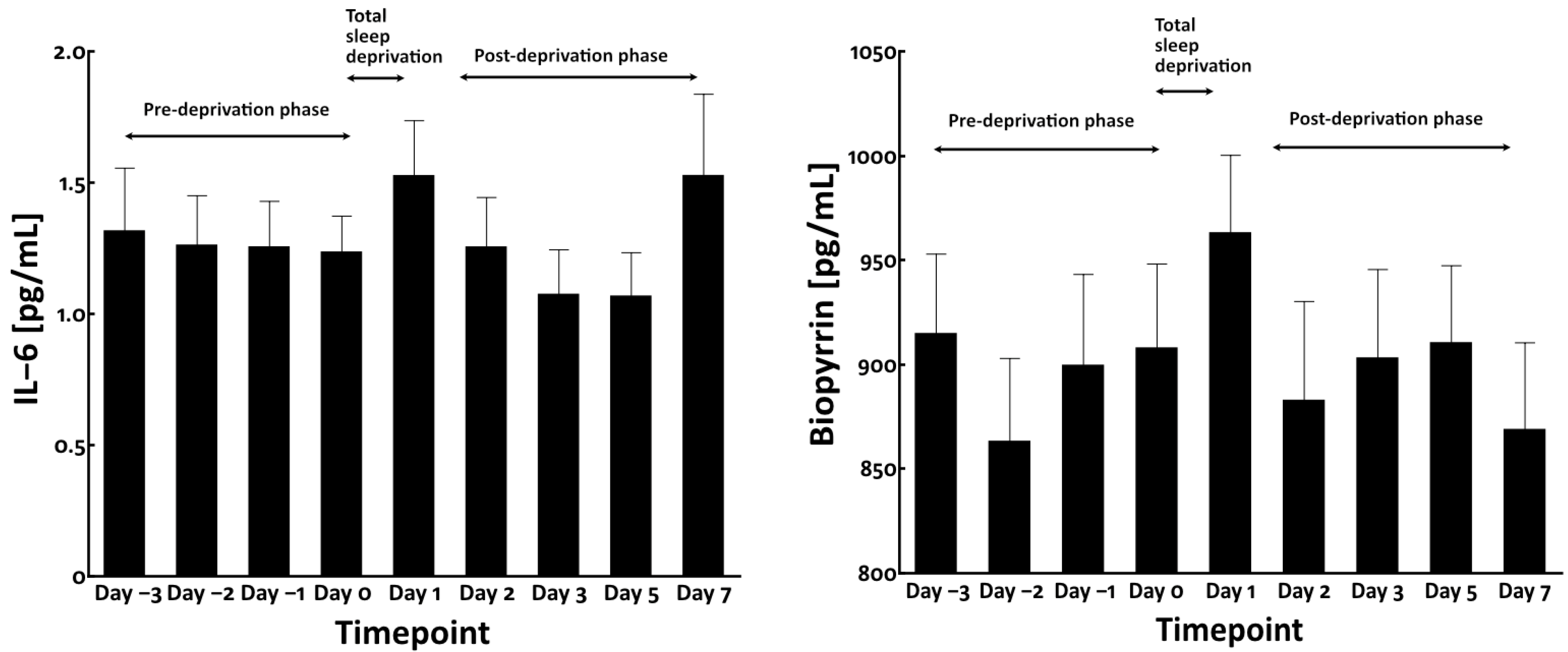
3.2. Total Sleep Deprivation Did Not Affect the Circulating Levels of Bilirubin and Carotenoids
3.3. Repeated Partial Sleep Deprivation Also Elevated Facial Skin Yellowness
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weinberg, M.K.; Noble, J.M.; Hammond, T.G. Sleep well feel well: An investigation into the protective value of sleep quality on subjective well-being. Aust. J. Psychol. 2016, 68, 91–97. [Google Scholar] [CrossRef]
- El-Sheikh, A. Children’s sleep, skin conductance level and mental health. J. Sleep Res. 2011, 20, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Okun, M.L. Biological consequences of disturbed sleep: Important mediators of health? Jpn. Psychol. Res. 2011, 53, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Archer, S.N.; Oster, H. How sleep and wakefulness influence circadian rhythmicity: Effects of insufficient and mistimed sleep on the animal and human transcriptome. J. Sleep Res. 2015, 24, 476–493. [Google Scholar] [CrossRef] [PubMed]
- Porkka-Heiskanen, T.; Zitting, K.M.; Wigren, H.K. Sleep, its regulation and possible mechanisms of sleep disturbances. Acta Physiol. 2013, 208, 311–328. [Google Scholar] [CrossRef]
- Kronholm, E.; Partonen, T.; Laatikainen, T.; Peltonen, M.; Härmä, M.; Hublin, C.; Kaprio, J.; Aro, A.R.; Partinen, M.; Fogelholm, M.; et al. Trends in self-reported sleep duration and insomnia-related symptoms in Finland from 1972 to 2005: A comparative review and re-analysis of Finnish population samples. J. Sleep Res. 2008, 17, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Knutson, K.L.; Van Cauter, E.; Rathouz, P.J.; DeLeire, T.; Lauderdale, D.S. Trends in the prevalence of short sleepers in the USA: 1975–2006. Sleep 2010, 33, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.I.; Jung, Y.; Lee, M.; Kim, J.; Kim, B.J.; Suh, B.F.; Kim, E. Evaluation of changes in skin characteristics due to the poor quality of sleep caused by smartphone usage. J. Cosmet. Dermatol. 2022, 21, 1656–1665. [Google Scholar] [CrossRef]
- Kalimo, R.; Tenkanen, L.; Härmä, M.; Poppius, E.; Heinsalmi, P. Job stress and sleep disorders: Findings from the Helsinki Heart Study. Stress Med. 2000, 16, 65–75. [Google Scholar] [CrossRef]
- Berset, M.; Elfering, A.; Lüthy, S.; Lüthi, S.; Semmer, N.K. Work stressors and impaired sleep: Rumination as a mediator. Stress Health 2011, 27, e71–e82. [Google Scholar] [CrossRef]
- Reyner, L.A.; Horne, J.A. Gender- and age-related differences in sleep determined by home-recorded sleep logs and actimetry from 400 adults. Sleep 1955, 18, 127–134. [Google Scholar]
- Axelsson, J.; Sundelin, T.; Ingre, M.; Van Someren, E.J.; Olsson, A.; Lekander, M. Beauty sleep: Experimental study on the perceived health and attractiveness of sleep deprived people. BMJ 2010, 341, c6614. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.A.; Kim, E.J.; Kang, B.Y.; Lee, H.K. The effects of sleep deprivation on the biophysical properties of facial skin. J. Cosmet. Dermatol. Sci. Appl. 2017, 7, 34–47. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.I.; Lee, M.; Han, J.; Kim, J.; Kim, A.R.; An, J.S.; Park, J.O.; Kim, B.J.; Kim, E. A study of skin characteristics with long-term sleep restriction in Korean women in their 40s. Skin Res. Technol. 2020, 26, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Oyetakin-White, P.; Suggs, A.; Koo, B.; Matsui, M.S.; Yarosh, D.; Cooper, K.D.; Baron, E.D. Does poor sleep quality affect skin ageing? Clin. Exp. Dermatol. 2015, 40, 17–22. [Google Scholar] [CrossRef]
- Fang, B.; Card, P.D.; Chen, J.; Li, L.; Laughlin, T.; Jarrold, B.; Zhao, W.; Benham, A.M.; Määttä, A.T.; Hawkins, T.J.; et al. A potential role of keratinocyte-derived bilirubin in human skin yellowness and its amelioration by sucrose laurate/dilaurate. Int. J. Mol. Sci. 2022, 23, 5884. [Google Scholar] [CrossRef]
- Han, J.Y.; Kim, E.J.; Lee, H.K.; Kim, M.J.; Nam, G.W. Analysis of yellowish skin color from an optical image and the development of 3D Skin Chroma Diagram(™). Skin Res. Technol. 2015, 21, 313–318. [Google Scholar] [CrossRef]
- Valkenet, K.; Veenhof, C. Validity of three accelerometers to investigate lying, sitting, standing and walking. PLoS ONE 2019, 14, e0217545. [Google Scholar] [CrossRef] [PubMed]
- Cabanas-Sánchez, V.; Esteban-Cornejo, I.; Migueles, J.H.; Banegas, J.R.; Graciani, A.; Rodríguez-Artalejo, F.; Martínez-Gómez, D. Twenty four-hour activity cycle in older adults using wrist-worn accelerometers: The seniors-ENRICA-2 study. Scand. J. Med. Sci. Sports 2020, 30, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Takiwaki, H.; Hillebrand, G.G.; Arase, S. Development of a digital imaging system for objective measurement of hyperpigmented spots on the face. Skin Res. Technol. 2002, 8, 227–235. [Google Scholar] [CrossRef]
- Miyamoto, K.; Dissanayake, B.; Omotezako, T.; Takemura, M.; Tsuji, G.; Furue, M. Daily fluctuation of facial pore area, roughness and redness among young Japanese women; Beneficial effects of Galactomyces ferment filtrate containing antioxidative skin care formula. J. Clin. Med. 2021, 10, 2502. [Google Scholar] [CrossRef] [PubMed]
- Logan, I.T.; Logan, R.A. The color of skin: Yellow diseases of the skin, nails, and mucosa. Clin. Dermatol. 2019, 37, 580–590. [Google Scholar] [CrossRef]
- Perrett, D.I.; Talamas, S.N.; Cairns, P.; Henderson, A.J. Skin color cues to human health: Carotenoids, aerobic fitness, and body fat. Front. Psychol. 2020, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xie, Y.; Li, Y.; Fan, X.; Xing, F.; Mao, Y.; Xing, N.; Wang, J.; Yang, J.; Wang, Z.; et al. Sleep deprivation and recovery sleep affect healthy male resident’s pain sensitivity and oxidative stress markers: The medial prefrontal cortex may play a role in sleep deprivation model. Front. Mol. Neurosci. 2022, 15, 937468. [Google Scholar] [CrossRef] [PubMed]
- Haack, M.; Sanchez, E.; Mullington, J.M. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep 2007, 30, 1145–1152. [Google Scholar] [CrossRef]
- Piber, D.; Cho, J.H.; Lee, O.; Lamkin, D.M.; Olmstead, R.; Irwin, M.R. Sleep disturbance and activation of cellular and transcriptional mechanisms of inflammation in older adults. Brain Behav. Immun. 2022, 106, 67–75. [Google Scholar] [CrossRef]
- Shibama, S.; Ugajin, T.; Yamaguchi, T.; Yokozeki, H. Bilirubin oxidation derived from oxidative stress is associated with disease severity of atopic dermatitis in adults. Clin. Exp. Dermatol. 2019, 44, 153–160. [Google Scholar] [CrossRef]
- Holding, B.C.; Sundelin, T.; Cairns, P.; Perrett, D.I.; Axelsson, J. The effect of sleep deprivation on objective and subjective measures of facial appearance. J. Sleep Res. 2019, 28, e12860. [Google Scholar] [CrossRef]
- Edwards, A.E.; Duntley, S.Q. The pigments and color of living human skin. Am. J. Anatomy 1939, 65, 1–33. [Google Scholar] [CrossRef]
- Sadiq, I.; Kollias, N.; Baqer, A. Spectroscopic observations on human pigmentation. Photodermatol. Photoimmunol. Photomed. 2019, 35, 415–419. [Google Scholar] [CrossRef]
- Yun, I.S.; Lee, W.J.; Rah, D.K.; Kim, Y.O.; Park, B.Y. Skin color analysis using a spectrophotometer in Asians. Skin Res. Technol. 2010, 16, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Sauvet, F.; Leftheriotis, G.; Gomez-Merino, D.; Langrume, C.; Drogou, C.; Van Beers, P.; Bourrilhon, C.; Florence, G.; Chennaoui, M. Effect of acute sleep deprivation on vascular function in healthy subjects. J. Appl. Physiol. 2010, 108, 68–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Leeuwen, W.M.; Lehto, M.; Karisola, P.; Lindholm, H.; Luukkonen, R.; Sallinen, M.; Härmä, M.; Porkka-Heiskanen, T.; Alenius, H. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS ONE 2009, 4, e4589. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsubara, A.; Deng, G.; Gong, L.; Chew, E.; Furue, M.; Xu, Y.; Fang, B.; Hakozaki, T. Sleep Deprivation Increases Facial Skin Yellowness. J. Clin. Med. 2023, 12, 615. https://doi.org/10.3390/jcm12020615
Matsubara A, Deng G, Gong L, Chew E, Furue M, Xu Y, Fang B, Hakozaki T. Sleep Deprivation Increases Facial Skin Yellowness. Journal of Clinical Medicine. 2023; 12(2):615. https://doi.org/10.3390/jcm12020615
Chicago/Turabian StyleMatsubara, Akira, Gang Deng, Lili Gong, Eileen Chew, Masutaka Furue, Ying Xu, Bin Fang, and Tomohiro Hakozaki. 2023. "Sleep Deprivation Increases Facial Skin Yellowness" Journal of Clinical Medicine 12, no. 2: 615. https://doi.org/10.3390/jcm12020615
APA StyleMatsubara, A., Deng, G., Gong, L., Chew, E., Furue, M., Xu, Y., Fang, B., & Hakozaki, T. (2023). Sleep Deprivation Increases Facial Skin Yellowness. Journal of Clinical Medicine, 12(2), 615. https://doi.org/10.3390/jcm12020615






